Investors & Media
Neuritek Therapeutics Inc. is a biopharmaceutical company committed to developing revolutionary treatments with the potential to transform the lives of patients and families suffering from psychiatric disorders. Neuritek is developing the first mechanism-based treatment for PTSD.
The IND for Phase 1 with the US FDA has cleared and the Phase 1 program has been successfully completed. Phase 2 PTSD is under preparation.
Dysregulation of GPCRs plays a key role in PTSD and leads to novel PTSD therapeutic approaches
Reduce the risk, time and cost to bring next-generation bio mechanism based treatment, treating the root cause of PTSD, to market for people suffering from Post-Traumatic Stress Disorder (PTSD).
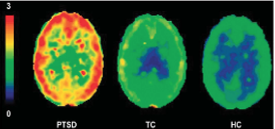
Abnormal G-protein coupled receptor-mediated anandamide signaling is implicated in PTSD.
TC: Trauma Control | HC: Healthy Control
Investor & Media Inquiries: info@neuritek.com
Addressing the Critical Need for Novel PTSD Treatments
PTSD is an unmet clinical need.
Post-Traumatic Stress Disorder (PTSD) remains a significant unmet medical need, affecting millions worldwide with debilitating symptoms that impair daily functioning and quality of life. While existing treatments provide relief for some, many patients struggle with persistent anxious arousal—one of the most distressing and treatment-resistant symptoms of PTSD. The need for novel, evidence-based therapies that offer both efficacy and safety is more urgent than ever. Unlike emerging psychedelic-based treatments, which face challenges around safety, regulation, and broad patient acceptability, there is a clear demand for innovative therapies grounded in rigorous science and proven pharmacological mechanisms.
Neuritek Therapeutics is advancing a next-generation approach to PTSD treatment through the development of a first-in-class FAAH inhibitor—a promising drug candidate designed to reduce anxious arousal by modulating key GPCRs (G protein-coupled receptors) involved in stress regulation. This targeted mechanism has shown potential to address hyperarousal symptoms without the psychoactive side effects or regulatory hurdles of psychedelics. With a strong focus on safety, tolerability, and clinical validation, Neuritek aims to deliver a transformative treatment option that can meet the critical needs of patients who currently lack effective solutions.